Manual Cell Counting in a Hemocytometer
|
Counting of cells (eukaryotic and especially mammalian cells) in a hemocytometer is an old but still valuable method to quantify the cell number per mL in a suspension. In the following paragraphs, we have outlined the most important points for counting with a hemocytometer. We also want to give hints where to start trouble shooting when problems with the counts arise. In general, many different kinds of chambers may be used to count cells. The Neubauer improved and the Thoma chamber, however, are the ones most often used in cell culture. A good overview of the subdivisions of the counting squares in different chamber types is given here (with German labels) Hecht Labor. |
 |
On this page, you find the following topics:
Short Protocol for an Optimal Counting in a Neubauer Chamber
Standardizing Counting in the Neubauer Chamber
Background Information on Cell counting
Use of Trypan Blue
Trypan blue is a vital stain. This means, intact cell don´t take up the dye and are not stained, while dead or damaged cell take up the dye and appear blue to lilac in the microscope. The stain is performed by mixing a trypan blue solution with the cell suspension to be counted in a reaction tube (e.g. 1:2 to 1:10 depending on the density of the cell suspension). After mixing, the solution is counted as usual in the hemocytometer. Non-stained cells = vital, healthy cells are counted and blue = dead, damaged cells are counted. Then the percent of vital cells is calculated = vital cells / (vital + dead cells). Depending on the cell type a normal range of vitality is documented (e.g. 90-95% for many cell lines, 60-70% for many T-cell lines, 50-69% for primary hepatocytes etc.). It is important to note, that trypan blue itself is slightly cytotoxic. After 5-10min it starts to kill the cells. Therefore, samples should not be "stored" for more than 5min before the count.
Everyone using a hemocytometer (e.g. Neubauer chamber) for cell counts should always perform a trypan blue stain. It does not take much longer, isn't expensive and is easy to perform. With the trypan blue stain, however, you gather more valuable information about your culture. The cell counts and vitality should be documented every time the cells are counted. Any change in the average cell number gained from a flask or the vitality should be noted and results should be checked.
Sterility
In order to count the cells, a sample has to be removed from the tube containing the cell suspension. This has to be performed in the biological safety cabinet (BSC). The next steps however, the mixing with trypan blue as well as the assembly of the counting chamber should be performed outside the laminar flow for safety reasons. Neither the trypan blue nor the chamber are sterile. Bringing them into the BSC unnecessarily enhances the risk of contamination.
Statistics
| The statistically optimal use of a standard hemocytometer is to perform a double sampling. For this, two independent samples are taken from the cell suspension and are separately filled into the two counting areas on the counting chamber. For assays in which cells are counted or when plating small cell numbers, this double count should always be performed. For other purposes, it is a widespread procedure to perform a single count only, which is less reliable. |  |
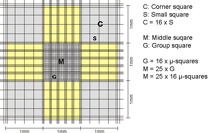
| For counts on both sides, it is intended to count all 4 large corner squares. The smaller the number of corner squares counted, the worse the statistical reliability. Of course, however, it takes longer to count 8 squares! As a rule of thumb, you always count at least 150 cells on your hand tally counter. If you have to count 4 corner squares to get to this number, then you should count 4. If you get 150 already in two squares, you might stop after 2.
For cell culture in GMP labs, you should check how many squares you have to count as a minimum during validation of the counting method. Don't forget to check results between personnel (at least three different persons should count). |
 |
Counting Method
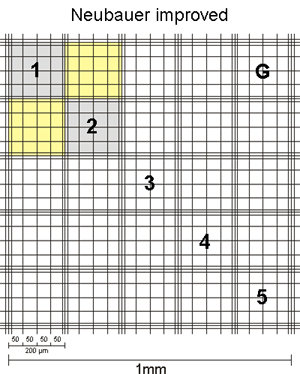
For normal mammalian cells, the corner squares of the hemocytometer are used to count. For smaller cells, such as erythrocytes, yeast or algae parts of the middle square (usually 5 group squares) are counted as these have smaller subdivisions and usually cell numbers are high.
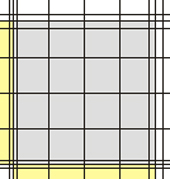
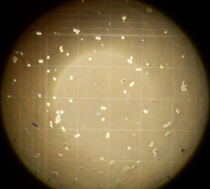
To have a better orientation during microscopy, the best option is to count with the 10 objective (=100x total magnification). Then, you can see the complete corner square with its 16 small squares. For the middle squares usually 20x or 40x objectives are used.
| Corner square Neubauer improved | Corner square in the microscope (10x objective) |
 |
 |
| Middle square Neubauer improved | Middle square Neubauer classic |
 |
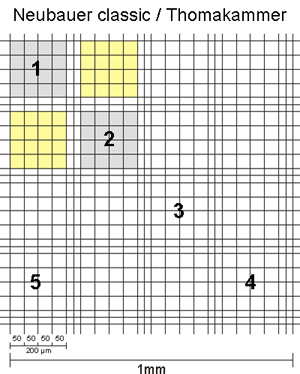 |
The sides of the corner squares as well as those of the middle square are each 1mm long. Therefore, the area of each corner square and of the middle square is 1mm2. When assembling the chamber by fixing the cover glass, a three-dimensional space is created. For almost all chambers, the height of this space = chamber depth is 0,1mm. The depth is also stated on the counting chamber. As a consequence, the volume to be counted for each corner square as well as the middle square is 0,1mm3 = 0,1µL. By multiplying the cells counted in one corner square by 104 (= 10.000) the cell number per mL is calculated as 104 * 0,1µL = 1mL.
The second number, listed on the chamber, states the area of 1 µ-square of the middle square (see above, usually 0,0025mm2). This number is given to allow for easy calculation of counts using parts of the middle square. As you can see, if you count all µ-squares your result is again cells in the same area as for the corner squares: middle square= 25 group squares with 16 µ-squares of 0,0025mm2 each = 25 * 16 * 0,0025mm2 = 1mm2.
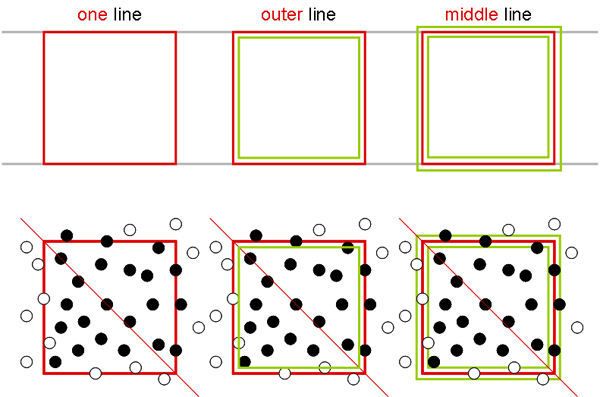
The corner squares of different chambers have different border lines. Some chambers have one, others two and the newer chambers have three outer boundary lines. In all cases, one of these lines is the one on which the decision to count or not count a cell is based. The others represent helper lines. In the picture below, the important defining lines are shown in red, while the helper lines are green.
In all cases, counting is performed in such a way that on two sides (in the pic top and right) cells touching the relevant line are counted, while on the other two sides (in the pic bottom and left) cells touching the relevant line are NOT counted.
Short Protocol for an Optimal counting in a Neubauer Chamber
Preparation
Suspension cells may be counted directly after mixing the culture and removing a sample. Adherent cells have to be trypsinized or accutased, centrifuged and resuspended before samples can be taken and counting can take place.
Materials
|
 |
Procedure
Within the biological safety cabinet (BSC)
- Mix cell suspension well
- Pipette 50 µL sample off with micropipette (20-200µL)
- Pipette sample into reaction tube 1
- Pipette another 50 µL sample off with micropipette (20-200µL)
- Pipette second sample into reaction tube 2
Outside the BSC
- Add 50 µL trypan blue to sample 1 and mix (= dilution 1:2)
- Take new tip
- Add 50 µL trypan blue to sample 1 and mix (= dilution 1:2)
- Humidify Neubauer chamber (by exhaling onto it or wetting with water)
- Place and fix cover slip, check for Newton's rings (rainbow-colored)
- Take new tip
- Add sample 1 to counting area 1 = top with pipette (approx. 20µL)
- Take new tip
- Add sample 2 to counting area 2 = bottom with pipette (approx. 20µL)
- Wait for 3 s
- Check whether cover slip is still fixed
- Place under the microscope
- Use 10x objective (inverted- or normal microscope)
- In the following steps, do not crash objective into chamber!
- Search top counting area and focus
- With 10x objective you should see a complete corner square
- Start with top left corner square and count all 4 corner squares
- Document results for 4 corner squares separately (lab book or form)
- Search bottom counting area
- Start with top left corner square and count all 4 corner squares
- Document results for 4 corner squares separately (lab book or form)
- Check values for homogeneity (are there large differences ? If yes, repeat whole procedure)
- Average results of all 8 corner squares
e.g. (51+54+50+52+53+51+53+52) / 8=52 - Calculate cell number per mL:
cell number / mL = average number per corner square( =52) * dilution factor from trypan blue (= here 2) *104 cells / mL
= 104 (result of: 52*2)* 104 cells/mL = 1.040.000 cells/mL = 1.04 Million cells per mL
Standardizing Counting in the Neubauer Chamber
Everyone who wants to standardize cell counting in a hemocytometer should take the following steps into account, check and define them in an SOP (standard operation procedure). The SOP should always contain a picture of the counting area subdivision and the dilution steps as here most of the misunderstandings arise:
- Time between mixing and sampling (cells settle fast)
- Dilution of the cell suspension (best is to write μL + μL)
- Pipettes and pipette tips (systems of a manufacturer - such as Eppendorf - are more precise than mixing systems)
- Check cover glass before and after applying the sample
- Number of squares counted. For precise counts, both sides have to be counted and also all 4 corner squares on both sides. For stock culture, it may be acceptable to do a single count or even count less than 4 squares.
- Exactly define how to count, especially with chambers that have more than 1 boundary lines
- Exactly describe the calculation formula. What has to be inserted when counting only 1 or 2 squares, with or without trypan blue etc. E.g. adding 5µL trypan blue to 45µL cell suspension is a 1:10 dilution of the cells!
Hemocytometers in GMP labs
Techniques used under GMP should be "state-of-the-art". For cell counting under GMP this means ussing an automated counting system. However, it is still possible to use hemocytometers in GMP labs. Nevertheless, a thorough validation of the method has to be performed in this case, with special emphasis on the inter-person variation of the results.


