Cell culture CO2 incubators: Overview and comparison
Which functions and specifications should an incubator have ?
Of course an incubator should regulate temperature, humidity and CO2 content!
But is that really all it should do ? No!
There are many features one should take into account especially when purchasing a new incubator. New and well-designed incubators can make cell culture easier, safer and more reliable because they have features not present or not of high quality in all incubators on the market. Our overview and comparison of incubator manufacturers will help you to ask the right questions and to judge which features are important for you. The most important criteria are:
|
|
How important these different features are for you mainly depends on whether you word in a research lab, an industrial or pharmaceutical testing or production lab or a lab where an ATMP (advanced therapy medicinal product) or TEMP (tissue engineered medicinal product) is produced. A ranking of decision criteria, that we find are a good basis for the comparison of manufacturers is listed at the very end of this page.
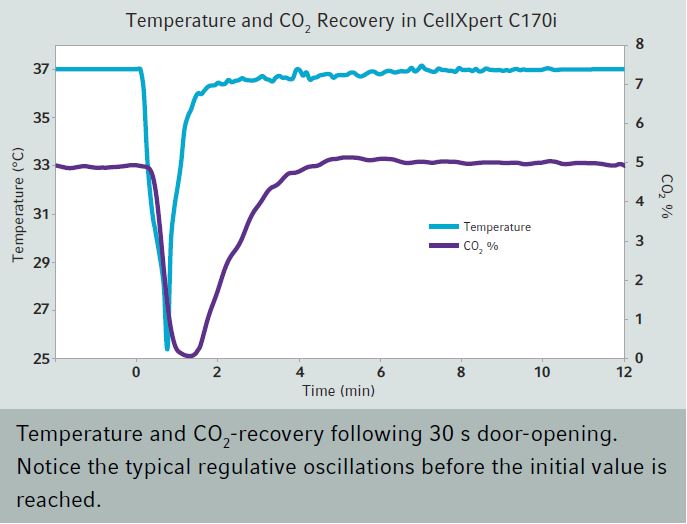
Temperature variation and recovery after door opening
Principles of incubator heating
|
For cell culture incubators, two main principles are used to heat the chamber and to keep the temperature more or less constant: + Water jacket incubators Advantages: better temperature stability, lower variation after door openings, longer temperature stability upon power failure Disadvantages: usually, high temperature sterilization is impossible, the incubator is heavier and harder to move, slow temperature recovery after very long door openings (> 15 min), Maintenance includes water exchange or refill - Air jacket incubators Advantages: high temperature sterilization is possible, the incubator is lighter and easy to move, faster temperature recovery after very long door openings (>15min), maintenance without water exchange Disadvantages: lower temperature stability, fast temperature drop upon door openings, short temperature stability upon power failure |
|
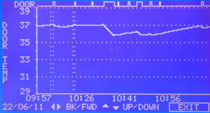
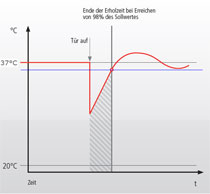
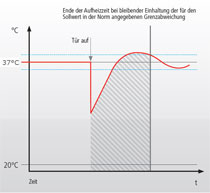
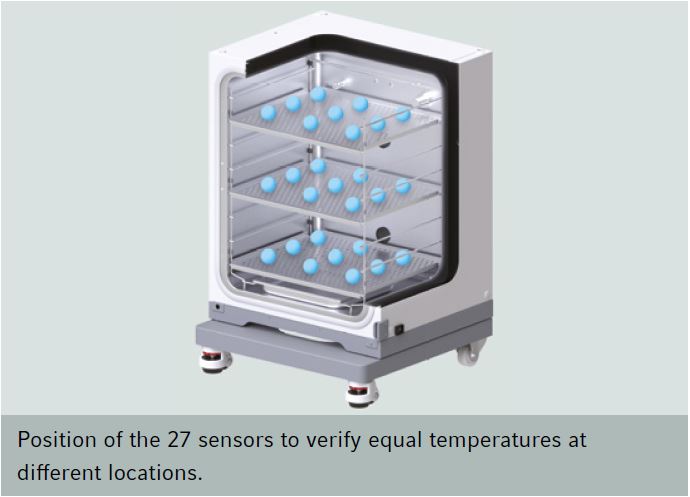
Recovery after door opening - measurement methodsFor the comparison of technical data from different cell culture incubator manufacturers, one can usually look up values collected during the mandatory normative type examination test (e.g. product characterization test, DIN Baumuster-Prüfung). Data is either given in the instruction manual, technical specification sheet or has to be inquired from the sales department. If you want to purchase an incubator, make sure you inquire all recovery times (temperature, humidity and CO2) from the vendor for comparison. German manufacturers and so some others validate the recovery of temperature after door opening (specification) of their incubators according to the DIN standard for incubators and list the recovery time in min. Here, it is important to know that this DIN standard (DIN 12880) was revised in 2007 and changes were made to the parameters and the analysis. The new version is DIN 12880:2007-05. Important for the comparison is that due to the changes in the measurement method, values acquired according to the new method give longer recovery times. Additionally, in larger incubators like those for cell culture more readings are required: 27 instead of 9 readings. The values are therefore, more precise. The different measurement set ups are depicted in the two schemes on the right. Top (old) and bottom (new). According to the old version, the temperature recovery time after a 30s door opening was reached as soon as the temperature once rose above the lower temperature fluctuation limit. As shown this results in very short recovery times. According to the new standard, the temperature recovery after a 15s door opening is reached only when the temperature fluctuation does not any more leave the temperature range between the lower and upper fluctuation limit. So on the one hand, the door opening time was shortened and on the other hand, the recovery is defined much more strictly. As a consequence, values acquired with these two methods can not be compared. In addition to this, one should check whether the incubator of interest has a separate door heating system (all six walls are heated). This reduces temperature gradients and condensation at the door. In comparison to the recovery of CO2 after door opening, the temperature fluctuation is less important. |
|
Temperature range of incubators
In addition to the above, one should compare the necessary temperature difference between the surrounding lab and incubator temperature. Incubators usually only are able to heat but not to cool their chambers. For a precise regulation of the inside temperature, they have to be located in a room that has a lower temperature. The minimum temperature difference they can counteract usually ranges from 5-7 Kelvin in a good incubator. In labs without air conditioning, this can become a critical factor during the summer months as large windows and direct sun light onto the incubator can easily lead to local temperatures of 30°C and above.
Principle of reaching 95% humidity
It is very important that no water evaporates from cell culture flasks, dishes or multi-well plates in the incubator. Otherwise the cells would be osmotically stressed or after some days even killed. Therefore, the humidity inside the incubator has to be maintained at about 95%. At the moment, there are three major ways in which this is achieved in cell culture incubators:
|
- Direct water fill onto the bottom plate (in our opinion a total no-go) + cheaper than other variants |
|
|
+ Indirect water fill into tub on bottom plate (state-of-the-art) + cheaper than the volatilization method below |
|
|
+ Volatilization of water (steam injection) (new but systems have to be thoroughly checked) + at first sight safest variant |
CO2 content and recovery after door openings
For the optimal growth of cells in an incubator, the CO2 content and its variation is the feature one should most look after. First, the incubator should keep the CO2 content as stable as possible and should recover very fast. Second, we should try to limit the loss of CO2 by keeping the number and duration of door openings as low as possible. For this, divided and well sealed doors as well as training of personnel are of crucial importance. Even though the DIN 12880:2007-05 standard does require the CO2 recovery measurement, one often has to inquire this separately from the manufacturer when comparing incubators.
Regulation of pH in cell culture media
|
The CO2 content of the incubator regulates the resulting pH in the used cell culture media. This means the CO2 content and the used medium are a system and work together to buffer the pH. When placing media in the incubator, an equilibrium between the sodium hydrogen carbonate solved in the medium and the CO2 content (in %) inside the incubator develops. The higher the CO2 content in the incubator the lower the pH of a given cell culture medium. Normal CO2 contents are 5%, 8,4% or 10%, depending on type of medium used. During the culturing period, the cells continuously secrete acid waste products which make the medium become more and more acidic. The carbonate buffering system slows the acidification down but after 2-3 days its buffering capacity is reached, the color turns from orange to more yellow and the medium should be changed. |
|
CO2 measurement types |
|
|
The CO2 content of an incubator is measured by a special sensor. This sensor may be either a TC (thermal conductivity) or IR (Infrared) sensor. When choosing a new incubator, you should pay attention that the incubator has an IR sensor of good quality (measurement time and precision). TC sensors are influenced by the fluctuation of temperature and humidity resulting from the door openings. Therefore, the TC measurement is much slower and the true values for the CO2 content are not displayed in real-time. As a consequence, the display may already show a CO2 content of 5% but the true percentage is still lower. For the cells, this results in a longer phase of pH basification which adds up especially when the doors are opened successively. IR sensor react much faster as the CO2 content is measured directly and independently of humidity and temperature. They show true values in real-time, make the recovery faster and thereby regulate the medium pH more efficiently and precisely. Another feature of the sensors is that some of them are built in such a way that they can remain in the incubator during a disinfection or sterilization routine. Other sensors have to be removed which takes more time and may be laborious. |
 |
Stability of CO2 content and recovery after door opening |
|
|
The stability of the CO2 content and the recovery after door opening are usually measured according to the standard DIN 12880 and are given in % and min, respectively. In contrast to the temperature, the CO2 content is measured at a single central reading point. It is important to note that the standard was revised in 2007. The actual version is now DIN 12880:2007-05. The revision changed the time of the door openings (15s vs. 30s) and way the recovery data has to be analyzed (see temperature). As a consequence, measurements according to the new version lead to a longer recovery time. So, to compare incubators always inquire the recovery time and measuring parameters. |
 |
Divided doors and reduced CO2 loss
General leakage of incubatorsAll incubators leak CO2. When the doors are closed, non-divided doors leak less than divided doors but in most labs this is counter-influenced by the number of doors openings per day. Compare to table below. Door openings and leakage in incubatorsWith each door opening, CO2 and humid, warm air are lost from the incubator chamber. The larger the door and the longer the door remains open, the larger is the loss and the longer does recovery take. Therefore, the door openings are crucial factors influencing the stability of growth conditions for the cells. They may become a problem, when many people share one incubator and the doors are opened successively with high frequency (e.g. university labs). In order to limit the extent of loss it is highly advisable to purchase an incubator with divided doors when several people share an incubator. Usually this results in a quite remarkable increase in price, but it's definitely worth it! You will later save money on CO2 and time on gas bottle exchange. In addition, the stability of surrounding conditions will make your results less variable. Always invest in divided doors in such cases! Depending on the manufacturer and model, incubators are offered with 2, 3, 4, 6 or 8 doors. As the doors of course change or limit the accessibility of the cell culture vessels inside, one should check the size and positioning of doors. For some incubators, 4 doors are a much better compromise than 6 or 8 viewed from both perspectives. In the photo on the right you see an old New Brunswick incubator with 4 doors which is now produced in a new and optimized design (see next section) by Eppendorf. |
 |
Leak tightness (sealing) of incubator doors
|
As stated above, all incubators leak gas. In general, divided doors leak more. However, depending on the number of door openings, this may play a large or no role compared to the loss caused by door openings. Compare the values given in the below table. This table shows some examples of leakage values for Binder incubators and the break-even calculation indicates how many door openings per day cause an incubator with divided doors to be the choice. In most labs divided doors are definitely the choice. For hypoxia conditions, one has to carefully calculate before deciding, as the large amount of nitrogen needed to wash out oxygen may lead to decide for a non-divided door. Another point where one can save money is the leak tightness of the door sealing and quality of the hinges of inner doors. Check the hinges for stability against vertical deformation and note where the doors are attached (only in the middle of the door is very instable). Compare the image on the right and the one above. The lower image shows the high quality door hinge of the new Eppendorf incubator. Also check whether the door closes well and whether its probable to not close the door correctly. Try to move the seals. Is it probable that they turn and create a leak ? For the outer door, check how the door is closed. Is it probable to not close the door be mistake ? Will it spring open ? |
 |
Gas consumption per day and per door opening in Binder incubators
|
CB 150/ CB 170 |
Standard glass door |
Divided |
Break- |
|
CB 210/ CB 220 |
|
Standard glass door |
Divided |
Break- |
|
|
Target |
Gasverbrauch |
g |
g |
|
Target |
Gasverbrauch |
g |
g |
|
|
|
5 % CO2 |
CO2 per day |
0,7 |
2,7 |
|
5 % CO2 |
CO2 per day |
1,0 |
5,0 |
|
|
|
|
CO2 per door opening |
13 |
4,5 |
0,2 |
|
|
CO2 per door opening |
16 |
3,9 |
0,3 |
|
30 % O2 |
O2 per day |
0,6 |
7,9 |
|
30 % O2 |
O2 per day |
0,6 |
17,3 |
|
|
|
|
O2 per door opening |
19 |
10 |
0,8 |
|
|
O2 per door opening |
33 |
9 |
0,7 |
|
5 % O2 |
N2 per day |
15 |
246 |
|
5 % O2 |
N2 per day |
12 |
540 |
|
|
|
|
N2 per door opening |
173 |
147 |
9,2 |
|
|
N2 per door opening |
300 |
148 |
3,5 |
|
1 % O2 |
N2 per day |
72 |
901 |
|
1 % O2 |
N2 per day |
53 |
1972 |
|
|
|
|
N2 per door opening |
350 |
283 |
12,4 |
|
|
N2 per door opening |
640 |
405 |
8,2 |
|
0,2 % O2 |
N2 per day |
125 |
1480 |
|
0,2 % O2 |
N2 per day |
207 |
3712 |
|
|
|
|
N2 per door opening |
452 |
388 |
21,0 |
|
|
N2 per door opening |
929 |
677 |
13,9 |
Surface material of the inner incubator chamber
An important feature for the ease and efficiency of cleaning (DIN and GMP) is the chamber surface material. Three types are currently on the market:
+/- Copper chamber and trays (bactericidal surface, but hard to clean as the corroded surface is very rough, expensive), not allowed in GMP production labs, not advisable elsewhere
+ Stainless steel (easy to clean as it has a very smooth surface, ATMP, TEMP as well as testing and production labs should choose this or stain-less steal copper alloy
++ Copper/stainless steel alloy (also bactericidal according to PHCbi (former Sanyo and Panasonic) but not corroding and therefore combining cleanability and bactericidal effects
Due to the cleaning efficiency and the prize the clear favorite is stainless steel for all research labs in our opinion. Safe the money on the alloy and instead buy divided doors! For ATMP and other GMP production the copper alloy is an interesting feature to additionally reduce the rsik of contamination.
Cleaning of incubator chambers (risk and effort)
|
This is a feature that initially is often ignored. However, efficient cleaning of the incubator is essential for contamination prevention and the easier this is achieved the smaller the risk of germ growing in the incubator. If we are honest, no one likes to clean the incubator. Especially not when racks and plates have to unscrewed, have corners and angles. So, the line of decision is clear - less is definitely more in this respect. When buying an incubator and when cleaning, think about two points:
|
 |
Ease of cleaning: what to look for ?
- Inner chamber without welding seams = drawn-out stainless steel (a MUST for ATMP and TEMP)
- Rounded corners
- Doors without welding seams
- Lowest amount of inner rack parts, no shelves screwed to inner chamber, no ventilator
- Easy disassembly of inner chamber parts for cleaning
In our opinion, the best inner construction design has been available from Binder. Now many other manufacturers offer this "no-plenum" design (Panasonic, 2019/2020 Eppendorf model, Ebert). The anti-plenum design is unbeaten as it has no inner parts except for the trays. The current Eppendorf Galaxy incubators also have a inner design that is fairly easy and fast taken apart for cleaning.The worst are Thermo and some asian brands where you need a tooth brush for cleaning.
Decontamination strategies inner chamber
Here, the state-of-the-art technology is by now an automated decontamination or even sterilization feature (sterile in place, SIP). Depending on the intended use of the incubator, one has to pay attention to the limits of these two hygiene measures as well as differences between manufacturers. Method, temperature and duration vary:
|
Heat sterilization according to or better than standards (ISO) and pharmacopoeias (for ATMP and TEMP advisable) |
|
Decontamination but not sterilization (sufficient for research and assay labs, pharma QC) |
|
So far, only available from Panasonic. H2O2 (Hydrogen peroxide) is a trend in pharma labs for efficient, especially sporocidal cleaning. Accordingly, it is in line with new developments that this sterilization method now appears for incubators and laminar flow cabinets. The major advantage for use in incubators is that the decontamination is rather fast (3h). As in most labs the long duration is the reason why the decontamination is performed only 1-2 times per year, this would allow for performing it more often. In research labs, cells could be left in the bench (closed) for the duration and afterwards recover over the weekend. After the procedure, small remains |
Sterilization: Requirements according to standards and pharmacopoeias
The terms sterilization and decontamination are differently defined in international standards and pharmacopoeias in Europe, USA, Japan etc. Disinfection is a so-called germ reduction by 3-5 log units (1:1.000 - 1:1:100.000 bacteria would survive). Sterilization starts at a germ reduction of 6 log units and above (1:1.000.000 bacteria would survive). Depending on the intended use or process, either disinfection or sterilization have to be ensured and validated.
| Standard or Pharmacopoeia | Temperature | Duration |
| European Pharmacopoeia | 160 °C | 120 min |
| US Pharmacopoeia | 170 °C | 120 min |
|
DIN EN 556 |
160 °C 180 °C |
120 min 30 min |
Additional Decontamination strategies (Air)
Several manufacturers offer incubators where all in-going gas (air and CO2) is filtered via HEPA filters. Even though each opening of the incubator door allows non-sterile air to enter the incubator, this is an additional risk mitigation feature and therefore, positive. However, filters are not cheap and they have to be replaced which means additional control and maintenance. In many cases, the use of filters also makes a ventilator necessary. This might be a contra-productive risk of contamination and vibration.
In summary, we think that air filtration is a feature that should be taken into account for ATMP, TEMP and IVF labs if available without ventilator. All other labs, especially university labs definitely do not need filtration.
Sockets and cable ports in the inner chamber
|
Another add-on in some incubators is the possibility to order the incubator with sockets or cable ports. This is a big advantage when you plan to perform new assays with equipment placed in the incubator like impedance measurements for cytotoxicity or migration or life cell imaging (video microscopy) with small microscopes that fit in the incubator like the JuLI. If you don't have a socket or cable port, cables have to be squeezed through the inner and outer doors (image on the right). This results in CO2 leakage and produces gradients inside the incubator with in turn may influence your assays or the other cells growing in the incubator and increases your use of CO2 . Cable ports can be sealed better than the doors and sockets circumvent the problem completely. Usually they are integrated in the chamber sides and have to be ordered with the incubator as they can not be installed later on. Not all manufacturers offer this feature. Binder does and additionally, you can also decide where you want it to be positioned (surcharge). |
 |
Vibrations (e.g. ventilators and pumps)
Ventilators and pumps may produce vibrations that might disturb your experiments or cell culture. They are true No-Gos for experiments like colony formation assays, stem cell and spheroid cultures. Additionally, they might influence adhesion of cells plated in flasks and multi well plates, producing unwanted patterns and distribution over the vessel surface.
This is the second reason -besides the contamination risk -, why we do not recommend incubators with ventilators. Additionally, the general standards for incubators does not demand any measurement in this respect. Therefore, one has to inquire this from the manufacturer if at all available.
Additional O2 control
Labs working on oxidative stress might want to consider the additional possibility to regulate oxygen by pumping nitrogen into the incubator. Many experiments over the last decade have shown, that the physiological oxygen partial pressure (physoxia) in tissue is way below the 21% in normal air (approx. 19-20% in the incubator) and therefore, resemble the situation currently named hypoxia (3-7%). The trend to lower the oxygen content moves forward only slowly but this type of experimental setup will surely become more important in the near future in this field as it has been clearly shown that e.g. wound healing, inflammation or neuronal cell death are strongly influenced by oxygen concentration.
Qualification and validation: service support
In GMP and GLP labs, as well as labs producing ATMP and TEMP, all quality relevant equipment has to be qualified and methods have to be validated. This starts with the purchase of the equipment where according to the QbD concept (quality by design) a URS (user requirement specification) has to be compiled. The URS lists all features and their acceptable ranges that the user wants to ensure. The manufacturer of choice would then reply to the URS commenting the URS specifications and listing the individual ranges they can ensure. For larger installations, a FAT/SAT (factory or site acceptance test) has to be performed. When the incubator is installed it has to be qualified in the lab of use according to the VMP (validation master plan). The 4Q qualification method includes DQ, IQ, OQ und PQ (design qualification, installation qualification, operational qualification and performance qualification). It is rather laborious and lots of paper has to be produced. In order to limit the hassle for customers, many manufacturers now offer validation support. This can range from offering documentation and performance templates to on-site validation up OQ or even PQ phases. Prizes and extend of services differ greatly. Ask for all material provided as some vendors also offer risk analyses or QbD documents now.
Manufacturer overview and where to order incubators
Below we are continuously collecting data for the comparison of incubator manufacturers and and overview of available features that are meant to help you decide which incubator is the best for you. In the very last section we have summarized a ranking of features for different types of labs. If you have additional comments or information, please contact us by mail or phone. User experience is always important!
Eppendorf (formerly New Brunswick incubators)
 |
|
| Production sites | Germany |
| QMS | ISO9001:2008 |
| Model | CellXpert C170 / C170i (170 L, successor of Galaxy models) |
| Contamination prevention (GMP relevant) |
|
| Ventilator | + No. |
| Vibrations | + No or little. No ventilator, no fan in air jacket. |
| Jacket |
Direct heating system of all 6 sides (includes door). Condensation trap in water tub (due to own heating circuit). |
| Door heating | Yes. |
|
Surfaces (GMP relevant) |
Inner chamber completely drawn-out stainless steel, no welding seams. Small number of inner parts easy to disassemble in 40 s for cleaning. Copper optional. |
Number of inner doors (indirectly GMP relevant) |
1, 2, 4, 8 (depending on model). |
| Door hinges | Very stable and attached over the complete height of the doors in new models. Hinge position (right/left) changable. |
| Temperature recovery (GMP relevant) | < 5 min (30 s door opening). |
| Temperature range |
4°C above RT up to 50°C. Several temperature sensors. |
|
Temperature stability (GMP relevant) |
Deviation from 37°C: +/- 0,1K measurement precision of control, +/- 0,3K homogeneity or uniformity over chamber. Measurement according to DIN12880 (German industrial standard). |
Humidity control (GMP relevant) |
Removable tub (stainless steel) with condensation trap (own heating circuit). Humidity control optional. |
| CO2 range | 0.2-20%. |
|
CO2 recovery (GMP relevant)
|
< 5 min (30 s door opening). |
| CO2 stability (GMP relevant) | Deviation from 37°C: +/- 0,1 % measurement precision of control, +/- 0,2 % homogeneity or uniformity over chamber. |
| Sterilization / Disinfection (GMP relevant) | Depending on the model, 140 to 180°C automated disinfection with downloadable protocol. |
| Decontamination duration (indirectly GMP relevant) | Incl. heating, decontamination and cool down: 14h. |
| Sensors (GMP relevant) | 2-channel infrared sensor for CO2, low in maintenance. All sensors are sterilizable and may remain in the incubator for the automated decontamination routine. |
| Sensor location | Top-middle. Ensuring precise and realistic CO2 measurements. |
| HEPA-filter | Yes, in the gas tubing. |
| User management (GMP relevant) | Yes, it is possible to define users with different rights (admin, user, guest etc.). |
| Data logger (indirectly GMP relevant) | VisioNize® Software with solid state hard drive for long-term storage, 2x USB, Ethernet-interface. |
| Validation support (GMP relevant) | IQ/OQ optional (surcharge). |
| Cable ports and sockets inside | 2 cable ports built-in. |
| Oxygen control | Optional. |
| Energy consumption | 100W at 37°C. |
| Price range | Medium to high. Lower budget model available. |
| Known advantages |
|
| Known disadvantages |
|
| Additional Information | |
| Web site | Products |
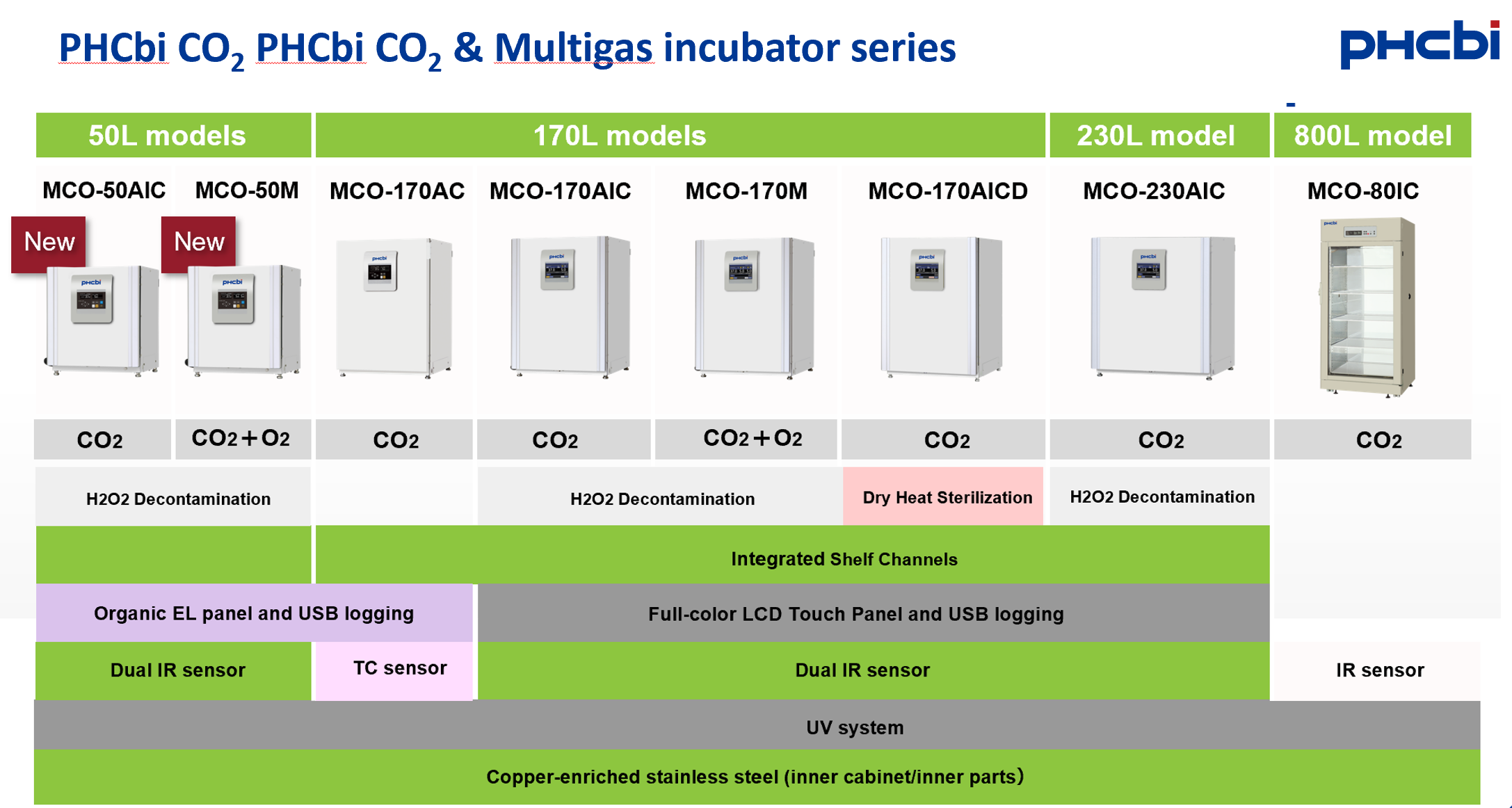
PHCbi (formerly Panasonic, Sanyo)
 |
 |
|
Production site |
Manufactured in Indonesia (complete process), designed in Japan. |
|
QMS |
ISO 9001:2015, ISO 140001:2015 and ISO 13485:2016. Medical Device Certification. MCO-170AIC-PE, MCO-170AICD-PE, MCO-230AIC-PE, MCO-170M-PE series are certified as a Class IIa Medical Device (93/42/EEC and 2007/47/EC) for medical purposes of culturing cells, tissues, organs (ATMP, GMP, TEMP, tissue preparation, stem cells) and embryos (for in vitro vertilization). |
|
Contamination prevention (GMP-relevant)
|
|
|
Ventilator |
Yes, but no known contamination problems. |
|
Vibrations |
No data available. |
|
All CO2 and multi-gas incubators have an air jacket, except for the MCO-170AICD-PE (heat sterilization) series. The MCO-170AICD has melamine foam insulation, which provides high thermal insulation and excellent heat endurance. Melamine foam insulation limits heat dissipation during dry heat sterilization. This means that cell culture can continue uninterrupted in incubators stacked with those actively running sterilization. |
|
|
Door heating |
Yes. All CO2 and multi-gas incubators have a door heater which is part of the Direct Heat System. This system regulates temperature through three independent heating zones under microprocessor P.I.D. control. (P.I.D.: Proportional Integral Derivative) |
|
Surfaces (GMP-relevant)
|
All internal surfaces are made out of copper enriched stainless steel (InCusaFe®). Easy to clean incubator interior featuring fully rounded corners and integrated shelf supports. All interior components, including the air management plenum, humidity pan and fan assembly are easily removable without tools if required. When components are removed, all interior surfaces are exposed for conventional wipe down with e.g. 70% ethanol or incubator cleaning liquids. Integrated shelf support system for easy cleaning available for MCO-170AC-PE, MCO-170AIC-PE, MCO-170AICD-PE, MCO-170M-PE and MCO-230AIC-PE series |
|
No. of Doors (indirectly GMP-relevant)
|
All units have 1 outer door and 1 glass inner door, except for the MCO-170M-PE multi-gas and MCO-170AIC-PE series. Standard stainless steel inner door with 4 glass doors for the MCO-170M-PE multi-gas series, this inner door is also optional for the MCO-170AIC-PE series. |
|
Door hinges |
Outer and inner doors are - as a standard - hinged on the left, field is reversible to the right. Door gaskets are magnetic to avoid air leakage. |
| Doors lockable |
Electronic Door Lock. Automatic door lock with password protection is available as a standard feature for the MCO-170AICUVH-PE, MCO-170MUVH-PE, MCO-230AICUVH-PE, MCO-170AICD-PE and MCO-170AICUVD-PE and can be easily set up. Other MCO-170AIC-PE, MCO-170AICD-PE, MCO-170M-PE and MCO-230AIC-PE series are compatible with the optional electric door lock (MCO-170EL). |
|
Recovery T (GMP-relevant) |
Temperature recovery times are not tested according to DIN12880. Recovery times are published in our product flyers and brochures. Varies per model. |
|
Temperature range |
Range ambient +5°C to 50°C. The CO2 and multi-gas incubators do not have a cooling functions. Overheat protection is settable and standard. |
|
Temperature fluctuations (GMP-relevant) |
Direct heat & air jacket heating system with P.I.D temperature control; variation +/- 0.1°C. Temperature uniformity is +/- 0.25K. Test conditions: Ambient temperature 23°C, setting 37°C, CO2 5%, O2 5%, no load. See also specs table in brochure. |
|
Humidity system (GMP-relevant) |
No active control. All units have a humidifying tray which can be easily retrieved, cleaned and filled. Bottom heater can be set separately to increase or decrease RH% by engineer if requested. Humidity Level & Fluctuation RH% control is 95% +/- 5%. Condensation management with the ‘dew stick’ controlled by Peltier technology - condenses water vapor on its surface, which then drips into the humidifying tray, preventing unwanted condensation in the chamber and possible contamination. |
|
CO2 supply |
Incoming CO2 gas will be filtered by a HEPA filter before entering the incubator. CO2 gas can be set to 0.03 MPa(G)–0.1 MPa(G). (Recommended pressure: 0.03 MPa(G)). CO2 hoses are equipped as standard. |
|
CO2 recovery |
CO2 recovery times are not tested according to DIN12880. Recovery times are published in our product flyers and brochures. Varies per model. |
|
Sensors (indirectly GMP-relevant) |
All MCO-170 and MCO-230 series have a temperature and humidity protected (state-of-the-art, faster, modern) IR CO2 Sensor - single beam with dual sensors for continuous auto-zeroing to ensure CO2 accuracy. P.I.D CO2 control ensures a rapid recovery to CO2 set-point. CO2 range 0 to 20 %, variation +/-0.15 % at 37°C 5 % set point. MCO-5AC-PE, MCO-5M-PE and MCO-170AC-PE have TC sensors. All sensors will stay in their original position during H2O2 decontamination or heat sterilization. Calibration after decontamination is not required. Dual IR CO2 Sensor- single beam with dual sensors for continuous auto-zeroing to ensure CO2 accuracy. The sensor is temperature and humidity protected. MCO-170AC-PE, MCO-170AIC-PE, MCO-170AICD-PE, MCO-170M-PE and MCO-230AIC-PE series. Solid State Zirconia O2 sensor with P.I.D control for rapid recovery to O2 set-point in MCO-170M-PE and MCO-5M-PE series. |
|
Sterilization (GMP-relevant)
|
Heat sterilization; Sterilisation process will start after the entire inside of the chamber exceeds 180°C and runs for 60 minutes followed by the cooling down phase. H2O2 decontamination; full cycle in 3 hours. This is the fastest sterilization method in the market. See also data in brochures. |
|
Sterilization duration (indirectly GMP-relevant) |
Heat sterilization; full cycle is 11 hours. H2O2 decontamination; full cycle in 3 hours. |
|
Sensor position |
The sensor is placed inside a sensor box on the back of the unit. Sensor opening inside the incubator chamber is in the air duct. |
|
HEPA filter (indirectly GMP-relevant) |
Yes, to filter incoming CO2, O2 and N2 gas. |
|
Data logger (indirectly GMP-relevant) |
In general, the MCO-170 and MCO-230 series all have a USB Port for logging temperature, CO2, door open status and alarms. The standard USB port allows for convenient transfer of log data from a USB memory stick to a computer. Data is logged for approximately 1.5 months, using a 2-minute interval. (Settable range: 2~30 min.) Optional analogue interface (4-20mA) is also available. |
|
Validation support (GMP-relevant) |
IQ/OQ offered. Price upon request. |
|
Cable ports or sockets |
One 30mm diameter access port as standard on every unit. |
|
Oxygen |
Yes, called multi-gas incubator: standard for MCO-170M-PE series and MCO-5M-PE series. |
|
Energy consumption |
E.g. 100 W at 37°C. Dependable on model. Data available upon request. |
| Stackable |
Incubators can be stacked with optional stacking kit. |
|
Price range |
Varies per model. Information available upon request. |
|
Known advantages |
|
|
Additional Information |
Service and download page for more information. |
|
Website |
Product page incubators. |
Binder
 |
|
| Production site | Germany |
| QMS | ISO9001:2008 |
| Contamination prevention |
|
| Ventilator | + No. |
| Vibrations | No data available. |
| Jacket |
Air jacket. |
| Door heating | No data available. |
|
Surfaces
|
Inner chamber completely drawn-out stainless steal, no welding seams, rounded corners. NO inner parts except trays = highest cleaning ease. 3 or optionally more trays.
|
|
Number of inner doors
|
1, 4 |
| Door hinges | Stable but not over the complete height of the doors. |
| Temperature recovery | Fast recovery: 4min (after 30s door opening; new DIN12880:2007-05) |
| Temperature range | 5-7°C above RT up to 60°C |
| Temperature stability | Deviation from 37°C: +/- 0,1K measurement precision of control, +/- 0,3K homogeneity or uniformity over chamber. |
|
Humidity control
|
Removable tub (stainless steel) with special cool trap that limits condensation in the incubator to the water tub instead of walls or doors. Humidity 90-95%. |
| CO2 supply | Supply after homogeneous mixing with air via a special valve. |
| CO2 recovery |
Fast: 7min (after 30s door opening, new DIN12880:2007-05) |
| Sterilization | Automatic sterilization according to pharmacopoeias and standards for medical devices. 180°C, 120 min |
| Sterilization duration | Incl. heating, decontamination and cool down: 10h. |
| Sensors | Infrared sensor for CO2, low in maintenance. All sensors are sterilizable and may remain in the incubator for the automated decontamination routine. |
| Sensor placement | Top. Ensuring precise and realistic CO2 measurements. |
| HEPA-Filter | No. |
| Data logger | Data logger control via display and USB. Ethernet interface for connection to computer and real-time control using the Binder software Apt.com. |
| Validation support | Yes. Full IQ/OQ support, trained personnel, documentation included. (Surcharge) |
|
Cable ports and sockets
|
Cable ports are optional and may be ordered with special positioning (surcharge). |
| Oxygen | Optional. |
| Energy consumption | 100W. |
| Price niveau | Middle to high. |
| Known advantages |
|
| Known disadvantages | |
| Additional information |
More information is provided in the download center: https://www.binder-world.com/en/download-center We have tested the older CB150 some time ago and were very satisfied. An absolute surprise was the transport support. Specialized fright personnel only transports Binder incubators. They all receive special training on the incubators and do not only transport the incubators to the lab but also install them there. No stress, no worries, no: "only up to here and not further" - that was excellent! The inner design of the chamber proved really well thought-through and functional. As promised we didn't see any condensation on doors or wall. All doors are stable and especially the outer door handle avoids that the incubator remains or springs open by accident. |
| Web site | Products |
Memmert
 |
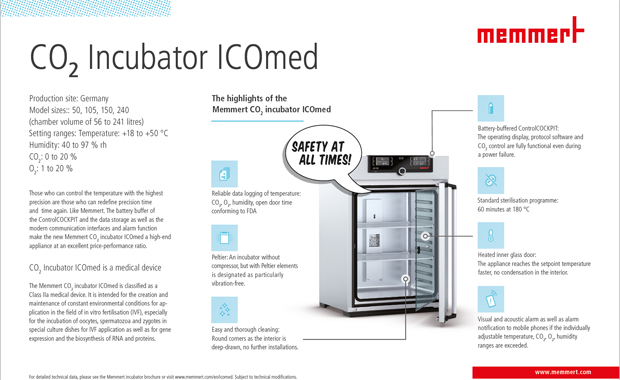 |
| Production site | Germany. |
| QMS | ISO9001:2008 / 12880:2007-05. One incubator is registered as a medical product class IIa: ICOmed. |
| Ventilator | + No. |
| Vibrations | One incubator without compressor but a Peltier element is listed as vibration reduced. |
| Jacket |
Large-area multi-function heating system. |
| Door heating | Yes. |
|
Surfaces
|
Deep-drawn, seamlessly welded. |
|
Number of doors
|
1, 2 or more depending on the model possible. |
| Door hinges | 2-point locking (compression door lock). |
| Recovery T |
Fast recovery at 37 °C (30 s door opening), duration varies for models: 50 = 3 min 105 = 4 min 150 = 5 min 240 = 6 min according to DIN12880:2007-05. |
| Temperature range | +18 to +50 °C. |
| Temperature fluctuations | Deviation from 37°C: +/- 0,1K. |
|
Humidity system
|
93 % rh +/- 2,5 %. Humidity limitation thanks to Peltier element. Integrated Humidity limiting control. |
| CO2 supply | Back wall. |
| CO2 recovery |
In 5 % CO2 incubators (30 s door opening): 50 = 6 min 105 = 6 min 150 = 7 min 240 = 7 min according to DIN12880:2007-05. |
| Sterilization | Sterilization according to DIN norm. |
| Sterilization duration | Standard sterilization program: 60 minutes at 180 °C (without removing the sensors). Cooling time not included. |
| Sensors | 2 Pt100 sensors Class A in 4-wire-circuit, mutually monitoring and taking over the performance at the same temperature value. |
| Sensor positioning |
Temperature sensor: in the back, at the top on the left side. CO2 sensor: in the back, half to 2/3 unit size left (depending on unit size). Humidity sensor: Position like CO2-Sensor. O2 sensor: in the back, half to 2/3 unit size right (depending on unit size). |
| HEPA-Filter | Yes: interior. |
| Data logger | AtmoControl Software, USB, Ethernet LAN. |
| Validation support | IQ/OQ/PQ offered. |
|
Cable ports an suckets
|
Yes: Silicon port. |
| Oxygen | Yes, optional |
| Energy consumption |
50 = 60 W at 37 °C 105 = 60 W 150 = 80 W 240 = 85 W |
| Price range | middle, high. |
| Known advantages |
|
| Known disadvantages | |
| Weitere Informationen | |
| Web site | Products and downloads. |
Additional manufacturers of cell culture CO2 incubators
Hettich (Products; lanched in 2016), Memmert (Incubators), Panasonic now PHCbi (Incubators), Thermo Fischer (Incubators, Heraeus), Dunn Labortechnik (Incubators), NuAir distributed by IBS Integra Technomara (Incubators), Ewald Innovationstechnik (Incubators), Infors AG (Incubators), Labotec GmbH (Incubators), Selutec GmbH (Incubators).
Purchase decision criteria for research labs
The price, for sure, is a major factor in almost all labs. However, sometimes it makes sense to pay a little more initially instead of having trouble and problems afterwards. This is our ranking of criteria for labs outside the regulated field of production.
- no ventilator unless it is easy to clean and disinfect (No Thermo incubator! Panasonic is very good)
- divided doors, good hinges (when frequently opening the doors)
- water tub
- as little inner construction as possible, no welding seams
- fast CO2 recovery after door opening
- price
- data logger without PC for maintenance and user control
- heat disinfection automated
Purchase decision criteria for pharma QC assay labs
Here, the main focus is consistency, reproducibility and low data variation. Additionally, most assay labs work according to ISO standards or under GMP/GLP. This is why we have a modified ranking of purchase criteria.
- no ventilator unless it is easy to clean and disinfect (No Thermo incubator! Panasonic is very good)
- divided doors, good hinges (when frequently opening the doors)
- water tub or steam injection (check system)
- data logger with PC for automatic control and documentation (validated unless you have an external sensor too)
- as little inner construction as possible, no welding seams
- cable ports if applicable
- rather two small incubators than one large one
- fast CO2 recovery after door opening
- price
- heat sterilization =/> 180°C for 2 h to be prepared for state-of-the-art moving to this level
Purchase decision criteria for ATMP and TEMP labs
ATMP and TEMP are medical products and therefore, underlie strict control and have to be of high and reliable quality. They have to fulfill specification requirements and the technical equipment used in production has to be state-of-the-art technology. This is why some of the criteria here become a MUST:
- An incubator which is manufactured according to ISO13485 (medical devices) would be a very strong pro for authorities (compare to risk analysis). However, at the moment there are not many incubators that fulfill this (Panasonic, Memmert) and hence, it is not mandatory. This might change in time as quality is a running target.
- heat sterilization =/> 180°C for 2 h (State-of-the-art is required, SIP is preferred, sterilization is better than disinfection, not yet mandatory)
- no copper surface (hard to clean, not allowed), instead stainless steal or copper-alloy (PHCbi)
- as little inner construction as possible, no welding seams (State-of-the-art is required)
- data logger with PC for automatic control and documentation (validated unless you have an external sensor too)
- Control and user management has to comply with ISO standards
- rather two small incubators than one large one
- fast CO2 recovery after door opening (consistent quality, all deviations have to be monitored)
- water tub or steam injection (the latter might be safer but QbD has to be performed)
- no ventilator unless it is easy to clean and disinfect (No Thermo incubator! Panasonic is very good)