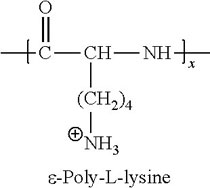
Coating (surface treatment) of cell culture plastic
Coating as an additional surface treatment stands for all additional modifications made to increase cell adhesion in addition to the standard plasma or corona treatment which is performed on all cell culture plastic by the manufacturer. Usually, coating is done with proteins or peptides.
Types of coating in cell culture
|
As described here, all cell culture plastic (T-flasks, multi-wells, dishes etc.) for culture of adherent cells is made from polystyrene and is surface-treated by the manufacturer in a specific chemico-physical way to allow for cell adhesion. However, this basic treatment does not even come close to the natural "surfaces" or extracellular matrix cells are growing on in tissues. This results in the option to tailor a cell culture model from very primitive and cheap up to highly complex and histotypic:
|
|
Why are coatings used ?
Coatings are more work or when pre-coated cell culture plastic is purchased more expensive as normal plastic. So, when should one use coated flasks or well plates ? For most basic experiments in 2D cell culture with cell lines, normal cell culture plastic is sufficient. However, there are several reasons that might make coating advisable or even absolutely necessary:
- Cells do not attach or do not well attach to normal surfaces (primary neurons, oligodendrocytes or hepatocytes as well as some cell lines as HEK293, OLN)
- The coating helps to avoid cell loss due to washing steps in assays (HEK293, OLN93, HepG2)
- The specific coating protein supports differentiation of cells (neurons, glia, hepatocytes, keratinocytes)
- Without coating the cells become senescent or dedifferentiate (MSC, hepatocytes)
- On specific surfaces, the cells develop a more complex morphology (glia, neurons, HepG2, hepatocytes, keratinocytes)
- Cells polarize only when cultured on a specific surface (HepG2, hepatocytes)
- Cells are culture in serum-free media and can't survive, proliferate or adhere without coating (s.u.) (glia, MSC, stem cells)
- The research focus is highly complex and can only be answered in a true 3D model (skin models, cartilage, bone, spheroids, angiogenesis)
In general, all cells would prefer to grow on a coated surface as this is more natural and allows for better adhesion. This means, normally the experimenter decides on the basis or the research focus and/or necessity. As for unpretentious cell lines there is no necessity to coat, cheaper non-coated plastic is used. For pretentious cell lines and especially primary cells, coating is helpful or even indispensable. No matter what we decide for, we always have to keep in mind that the closer the cell culture model is to the natural tissue situation, the easier it is to translate results for in vitro to to in situ.
Application examples for different coatings
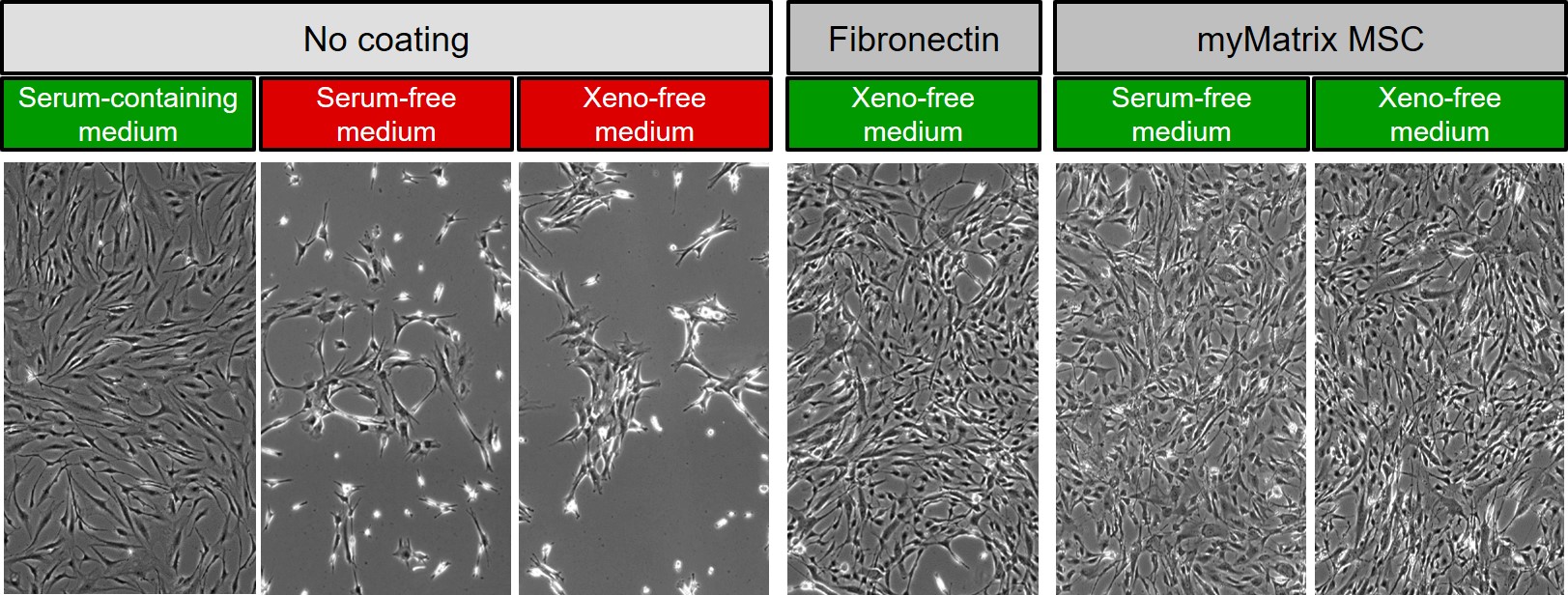
Serum-free or xeno-free cell culture of MSCs (mesenchymal stem cells)
In the following image of denovoMATRIX, different cultures of mesenchymal stem cells (MSC) are shown after culture on normal and coated surfaces with either FCS-containing, serum-free or xeno-free medium. The two combination labelled in red clearly show that with the change from serum-containing to serum-free medium, the necessity of surface coating arises. Due to the lack of serum proteins the pretentious MSCs can not any more attach to the cell culture plastic surface unless it is coated either with purified fibronectin or a synthetic replacement fro fibronectin manufactured by denovoMATRIX (myMARTIX).
|
© denovoMATRIX modified by InCelligence |
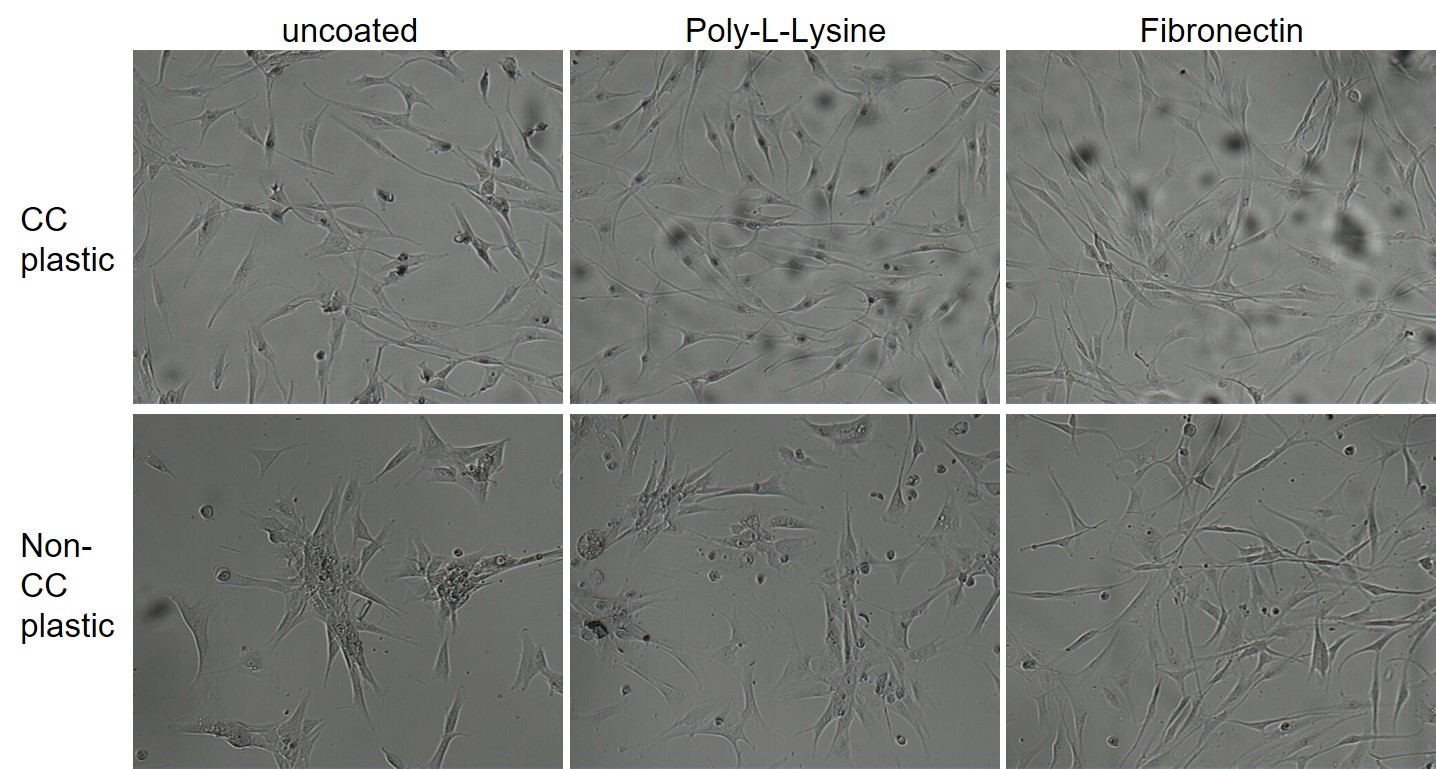
Culture of human fibroblasts on different surfaces with FCS-containing medium
The following images show normal human fibroblasts cultured in FCS-containing medium on different surfaces. The top row (normal cell culture plastic with or without additional coating) allows for good cell growth and the fibroblasts display a typical morphology. Only in the lower row (bacterial plastic surface) the effect of coating becomes obvious, as the fibroblasts can not well adhere to the non-treated, hydrophobic plastic. As a result one would judge that coating with FCS in the serum is unnecessary for human fibroblasts.
|
© InCelligence from course bioassays |
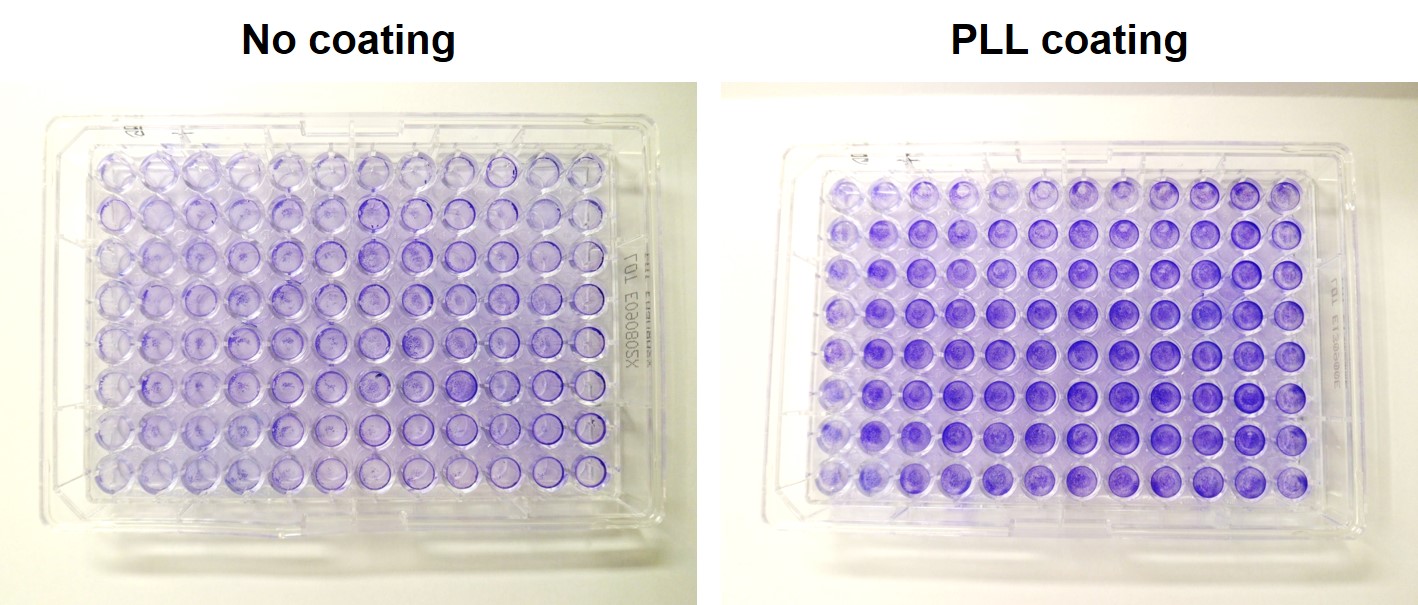
PLL-coating of 96-well plates help to avoid washing cell off in bioassays
In complex bioassays with many washing steps, it occurs quite often that the cells are partially or almost completely washed off while staining or during an ELISA. This might severely increase the standard deviation or in the worst case might make the whole assay non-measurable. In order to avoid these effects, well plates may be coated with PLL (easy, fast, cheap) for all cell lines or at least for such cell lines that are known to adhere not very well (HEK 293, OLN93). This helps to reduce cell loss at least partially or with the correct pipetting technique completely. The following photos show the effect of washing in an ELISA assay after identical cell numbers of HEK cells were plated on the same cell culture plastic but either with or without PLL-coating. Cells were visualized using a crystal violet staining protocol. The protective effect is easily seen on the right by the more intense color. The only exception is the very left row.
|
© InCelligence from course bioassays |
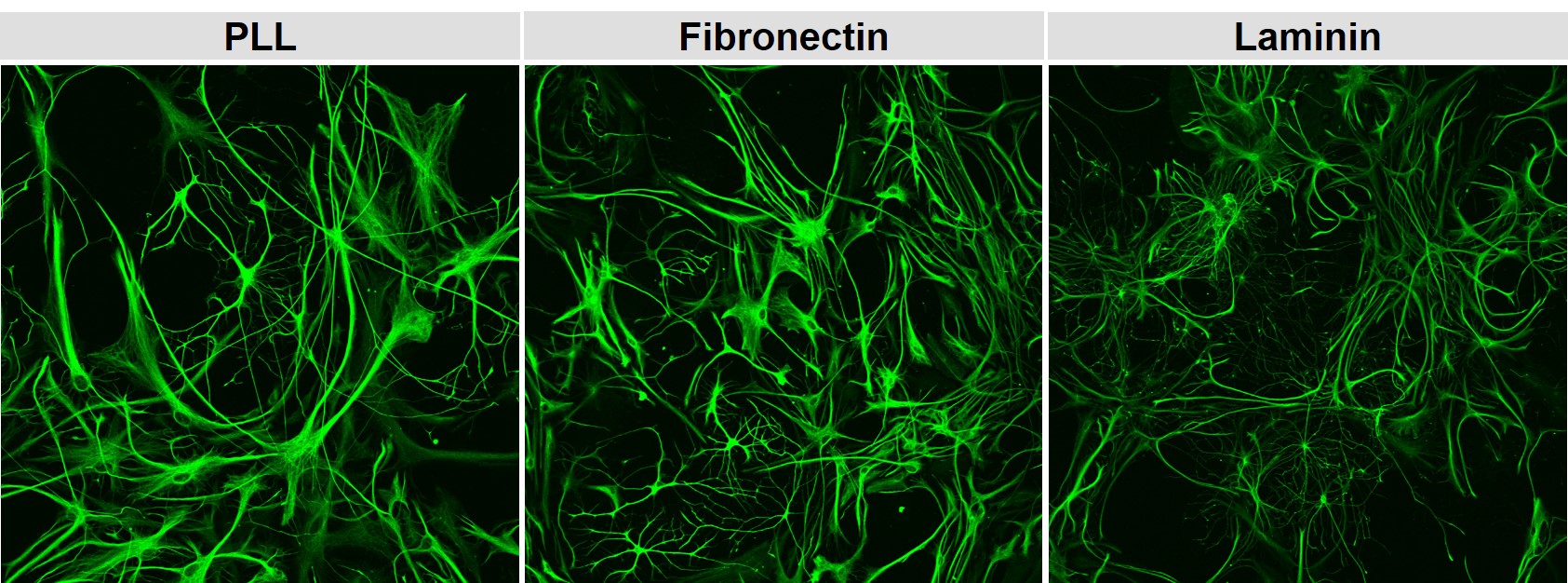
Extracellular matrix as a support for differentiation
For some cell types, the extracellular matrix helps the cells to orient themselves in which region or where in a tissue they are and helps them to detect who the neighboring cells are. These signals are detected by the binding of surface receptors (integrins) to the extracellular matrix. Intracellularly the binding elicits signalling cascades which then might contribute to proliferation, migration or differentiation. The images below show neonatal rat astrocytes (GFAP-stain) in a mixed glail culture on different coatings. Laminin triggers changes in the morphology of the astrocytes indication a different route of differentiation.
|
© InCelligence from Cell culture QA course |
Market overview: Coatings
The following table summarizes the current coatings offered on the market in addition to the standard ECM proteins by different manufacturers.
|
|
Ease of use (protocol free) |
Adhesion Ligand |
Oligo-saccharide |
Degradable |
Cell recovery/ Trypsination |
Chemical reaction free |
Growth Factor storage/ |
Chemically-defined |
Microscopy compatible |
Suitable for Stem cell culture |
Suitable for Cancer cell culture |
Suitable for Primary cell culture |
Suitable for screening |
|
|
Fibronectin |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
ECM protein |
||||||
|
Vitronectin |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
ECM protein |
|||||
|
Osteopontin |
▪ | ▪ | ▪ | ▪ | ▪ |
ECM protein |
||||||||
|
Collagen (I,IV,etc) |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
ECM protein |
|||||
|
Gelatin |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
ECM protein |
|||||||
|
Laminin |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
ECM protein |
||||||
|
Biolamina, recombinant Laminin |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
ECM protein |
|||
|
3D MATRIX, puramatrix |
▪ | ▪ | ▪ | ▪ | ▪ |
Peptide nanofiber hydrogel |
||||||||
|
Pbiogelx, biogelx powder |
▪ | ▪ | ▪ | ▪ | ▪ |
Peptide nanofiber hydrogel |
||||||||
|
ESI-BIO, Hystem |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Hyaluronate, collagen, Polyethylene glycol |
||||||
|
Trevigen, cultrex |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
EHS tumor extract |
||||||
|
East River BioSolutions, TissueSpec |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Tissue-specific extracts |
||||||
|
Ectica, 3DProSeed |
▪ | ▪ | ▪ | ▪ | ▪ |
Polymer |
||||||||
|
Corning, matrigel |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
EHS tumor extract |
||||||
|
Corning, Synthemax |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Vitronectin-derived peptide |
|||
|
Corning, Synthemax II |
▪ | ▪ | ▪ | ▪ |
Plastic microbeads coated with synthemax |
|||||||||
|
Corning, Osteo-assay |
▪ | ▪ | ▪ | ▪ | ▪ |
Crystalline calcium phosphate |
||||||||
|
Corning, Ultra-low attachment |
▪ | ▪ | ▪ | ▪ |
hHdrophilic uncharged polymer surface |
|||||||||
|
BRTI Life Sciences, Cell-Mate3D |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Hyaluronate -Chitosan hydrogel |
||||||
|
Pepgel, PGmatrix |
▪ | ▪ | ▪ | ▪ | ▪ |
Peptide hydrogel |
||||||||
|
Novamatrix, Novatach |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
RGD coupled alginate |
|||||||
|
Orla, OrlaExplorer-ECM |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
32 recombinant ECM proteins |
||||
|
Cellendes, 3D-Life |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Dextran PVA - PEG hydrogels |
|||||||
|
Amsbio, mimetix |
▪ | ▪ | ▪ | ▪ | ▪ |
PLLA electrospun fibres |
||||||||
|
Amsbio, iMatrix |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
E8 Fragment from laminin |
||||
|
Amsbio, MAPTrix |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Recombinant chimeric ECM proteins |
||||
|
Amsbio, Alvetex |
▪ | ▪ | ▪ |
Porous Polystyrene sheet |
||||||||||
|
Noviocell, PIC hydrogel |
▪ | ▪ | ▪ | ▪ | ▪ |
Thermoreversible polyisocyanopeptide hydrogel |
||||||||
|
Organogenix, NanoCulture Plate |
▪ | ▪ | ▪ | ▪ |
Low adhesion patterned plates |
|||||||||
|
Primorigen biosciences, StemAdhere |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Recombinant protein, human origin |
||||||
|
The well bioscience, Vitrogel 3D |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Polysaccharide hydrogel, non-covalent crosslink |
|||||||
|
Thermofisher, Algimatrix |
▪ | ▪ | ▪ | ▪ | ▪ |
Alginate gels |
||||||||
|
Thermofisher, CELLstart |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Human plasma extract |
|||||
|
Thermofisher, Geltrex |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
EHS tumor extract |
|||||
|
Lonza, RAFT |
▪ | ▪ | ▪ | ▪ | ▪ |
Collagen, RAFT (Real Architecture For 3D Tissue) |
||||||||
|
Global Cell Solutions, GEM |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Alginate microcarrier ferromagnetic beads |
|||||||
|
InSphero, GravityPLUS |
▪ | ▪ | ▪ |
Hanging drop ECM-free |
||||||||||
|
DenovoMATRIX, screenMATRIX |
▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
Glycosaminoglycan PEG-Peptide biomatrix |