Comparison Mycoplasma test kits for cell culture (research to GMP)
|
If you want to purchase a mycoplasma test kit, you may use this overview and comparison of manufacturers and kits as decision aid. Such a comparison is highly recommended as the prices and detection limits as well as specificity tremendously vary. There are many kits on the market and some clearly are much better than others. In addition, we also shortly outline the principles of the PCR and DAPI test methods for mycoplasma. |
 |
The following topics are covered on this page:
Mycoplasma detection and test kits
Species found in cell cultures
Mycoplasma detection methods
Mycoplasma detection can be achieved by several different test methods:
|
Among these methods are two that are most recommendable due to price, ease, specificity and sensitivity and these are the DAPI stain and PCR. A DAPI or Hoechst stain is easy and fast even though it is harder to read-out. The PCR test is the state-of-the-art technique as it combines - in the best case - ease of performance, speed, price, sensitivity and specificity. Below we have crudely outlined how - in a research lab - you can perform mycoplasma tests using one of these two methods.
PCR detection
In the PCR example, we show how to test an FCS batch that was newly purchased. First you prepare a known clean cell culture. To this, you add medium containing the new FCS batch. Now you grow and passage the cells (best without antibiotics) for at least a week to give the potential mycoplasma contamination time to grow. Longer culture periods would be safer. Then, you take a media sample from this culture. After boiling for approximately, 5min the sample is ready for testing using a PCR kit like the ones listed below. After 3-4 hours you get the result as bands on a gel.
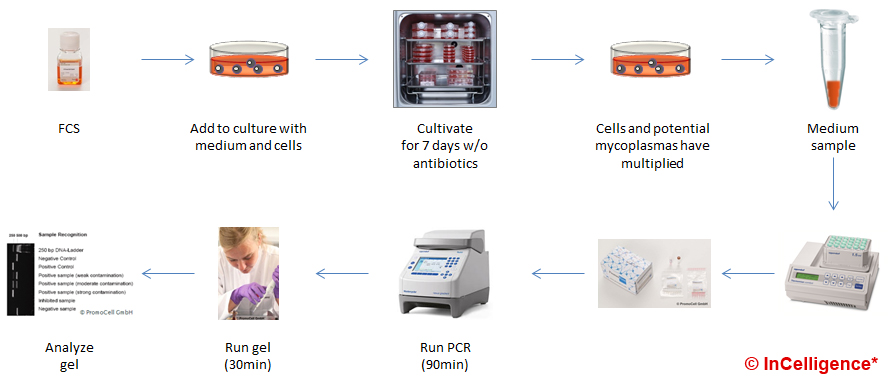
When performing a PCR detection of mycoplasmas in a GMP production surrounding, this very crude and basic protocol has to be changed in several aspects and controls have to be included. This is why research level kits have a true sensitivity of roughly 1.000 CFU/mL while GMP final product testing uses tests validated to have a sensitivity of 10 CFU/mL for all relevant species.
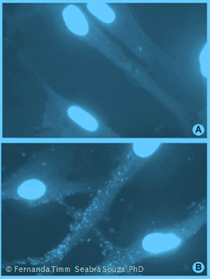
Detection by DAPI stain
The second example outlines the DAPI stain. You start off as described above with adding the test sample to a clean culture and culturing for at least 7 days. Then. you discard the medium, you wash and fix the cells with formaldehyde and stain them with DAPI or Hoechst solution. Now you can read out the result using a fluorescence microscope under the UV setting. As this method is much less sensitive it is better to prolong the culture period to 14-21 days in order to be really sure.

To use this test in a GMP production surrounding not much more than the cultivation period has to be changed. Of course controls have to be included.
Mycoplasma detection and test kits
| Supplier | Method/DL/GMP | Species / Test result type | Price | Remarks: Research kits |
| Example video |
PCR-Gel <1000-2500 CFU GMP: - |
28 (yes/no answer) =
|
<6,63 |
Positive control: yes
In our MOOC, we have used this kit to demonstrate the method of PCR in a Video . |
| Minerva |
PCR-Gel <1000-2500 CFU GMP: - |
28 (yes/no answer) =
|
<10,34 |
Positive control: yes |
| Sigma |
PCR-Gel <1000-2500 CFU GMP: - |
19 (yes/no answer) =
|
<9,96 | Positive control: yes Internal control: yes Simplified kit as described above with lyophilized reagents in tubes. LookOut® |
| Supplier | Method/DL/GMP | Species / Test result type | Price | Remarks: No internal control |
| R&D Systems |
Hybridization- ELISA ~15 - 2500 CFU GMP: - |
8 (yes/no answer) =
|
~4 | Positive control: yes Internal control: no With this kit you detect mycoplasma rRNA in a colorimetric ELISA. The assay cross reacts with Lactobacillus and therefore, is not mycoplasma-specific. In addition the sensitivity is lower. MycoProbe® |
| Agilent |
PCR-Gel n.a. GMP: - |
Description states "most" (yes/no answer, 5 identified) | ~9,8 | Positive control: yes Internal control: no This kit only contains the primers. No polymerase or master mix are included. After a PCR, 5 species can be identified in a restriction digest run on a gel. The product information does not state what is meant with "most" species. Inquire and compare to the lists below! The internal control provided is a mycoplasma DNA and therefore only serves as a PCR control. Mycoplasma Plus Primer set |
| Supplier | Method/DL/GMP | Species / Test result type | Price | Remarks: Research kits |
| Greiner BioOne |
PCR- Mikroarray <10 CFU GMP: + |
all known species (yes/no answer), (EuPharm 9 of 7-9) 40 (identifiziert) |
>560 |
Positive control: yes |
| Roche |
PCR-Gel <10 CFU GMP: + |
all known species (yes/no answer), (EuPharm ? of 7-9) | >500 |
Positive control: yes |
* Price is price per sample for a kit size ~ 25 samples.
Mycoplasma species listed in the EuPharm.:
The European Pharmacopoeia precisely lists how to test for mycoplasma contamination in sterile products and which species to use for validation and verification. Depending on what you test, the detection limit has to be 10-100 CFU per mL. Depending on the product, one of these control species is run as a positive control in all batch tests. Purified mycoplasma DNA should never be used as a GMP positive control as it does not realistically reflect the results obtained with intact mycoplasma as a positive control.
- A. laidlawii
- M. fermentans
- M. hyorhinis (where cell-culture enrichment is used, a fastidious strain such as ATCC 29052 is included)
- M. orale
- M. pneumoniae or M. gallisepticum
- M. synoviae (where there is use of or exposure to avian material during production)
- Mycoplasma arginini
- Spiroplasma citri (where there is use of or exposure to insect or plant material during production).
The most important and relevant species for culture
There are several test systems and test kits on the market. Several of these detect only a limited number of species. Make sure that the species below are in the detection range (specificity and sensitivity) of the kit you purchase. There are several kits that are cheap and have a wide range of detection.
- M. orale
- M. hyorhinis
- M. arginini
- M. fermentans
- M. salivarium
- M. hominis





